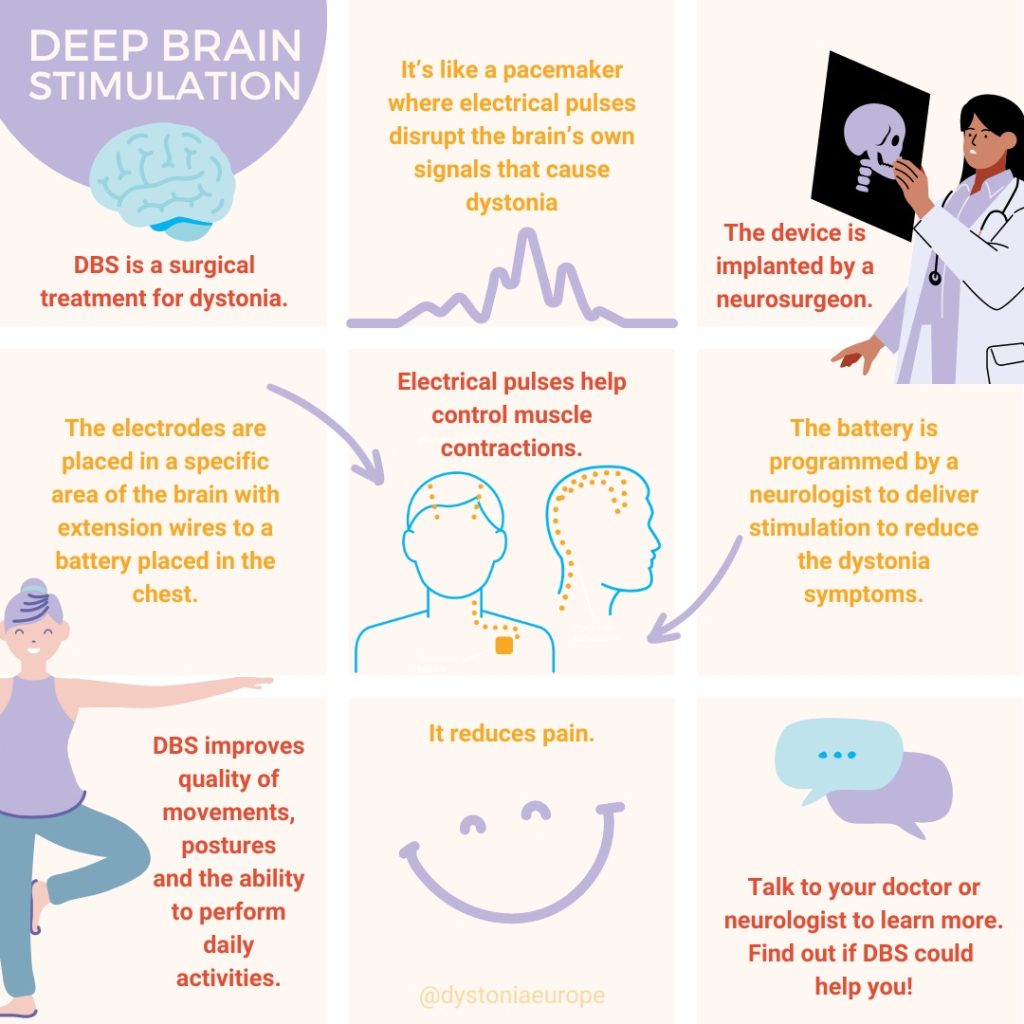
DBS treatment is reserved to those patients who, despite periodic injection with botulinum toxin, are still disabled and have poor quality of life due to cervical dystonia. For DBS surgery, two electrodes are commonly implanted in both sides in a deep structure of the brain named Globus Pallidus pars interna (GPi). Sometimes, other areas may be targeted by DBS for dystonia, such as the subthalamic nucleus (STN) and the thalamus (for patients with prominent head tremor). These electrodes are connected to a stimulator that is implanted underneath the skin in the chest wall. This works as a brain pacemaker which emits electrical signals to help control muscle contractions. Only a few patients are eligible for this treatment, but they may gain great benefit. Side effects include those associated to the neurosurgical intervention (brain haemorrhage) or to the implanted device (brain infection, malfunctioning of the device). These side effects are extremely rare. Moreover, the DBS device needs periodic review by a neurologist expert in DBS who will adjust the settings to improve symptoms of cervical dystonia, while avoiding stimulation-induced side effects (muscle contractions, speech problems).
Brochure Dystonia and DBS
For more information about the DBS treatment Dystonia Europe has developed a leaflet in collaboration with Professor Inger Marie Skogseid at Oslo University Hospital.
